Neuronal Connectivity Laboratory: Cytoskeleton
Yu-Chih Lin, Ph.D., Investigator
Structural stability of neurons
The human brain is composed of billions of neurons that have characteristic shapes, with elaborate neurites including axons and dendrites. In excitatory neurons, actin-rich cellular protrusions called dendritic spines extend from dendrites and serve as sites of synaptic contacts with other neurons. In GABAergic interneurons, the arborization of dendrites and axons determines the synaptic strength. Disruptions in the complexity of neurites and the stability of dendritic spines are hallmarks of almost all neurological conditions, including Autism Spectrum Condition (ASC). These structural defects in neurons often result in alterations of excitatory and inhibitory balance in the brain circuits by affecting the proper synaptic contacts among neurons. Changes at the cellular level of neurons consequently affect both cognitive and behavioral performance and contribute to the challenges of affected individuals and their families. Thus, understanding the mechanisms underlying the proper formation and maintenance of neurites and synapses will provide important insights to guide the development of new therapeutic interventions to ameliorate these conditions. We are particularly interested in how the regulatory mechanisms are distinct between excitatory and inhibitory neurons.
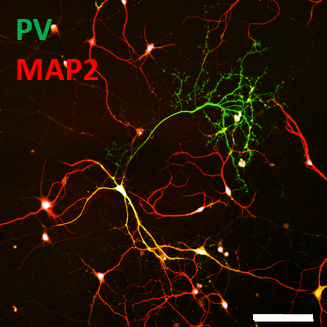
Figure 1. A parvalbumin-positive (PV+) GABAergic interneuron in culture shows simple MAP2-postive dendrite arbors (red; merge as yellow) and elaborated PV+ axonal branches (green). Scale bar is 100 µm.
The small RhoGTPase pathway
Several mechanisms, including trans-synaptic adhesion receptor coupling, synaptic activity, and trophic signals from the presynaptic compartment, play important roles in regulating the formation and stability of dendritic spines. Although their mechanisms of action are diverse, these signaling pathways often converge when they act on cytoskeletal regulators within the dendritic spine. We are interested in how cytoskeletal machinery is regulated to provide structural and functional stability to neurons, and how this regulation is disrupted in the disease state. Small RhoGTPases including Rho, Rac, and Cdc42 are central cytoskeletal regulators that control cell motility and morphology. Genetic mutations or dysregulation of small RhoGTPase regulators, including guanine-exchange factors (GEFs) and GTPase-activating proteins (GAPs), has been implicated in several neurological conditions including ASC. GEFs and GAPs determine the activity of small RhoGTPases and in turn regulate cytoskeletal dynamics in dendritic spines. Using neuronal cultures as model systems, we are combining high resolution imaging techniques and biochemical approaches to resolve how cytoskeletal machineries, including the small RhoGTPase pathway, are altered in ASC.
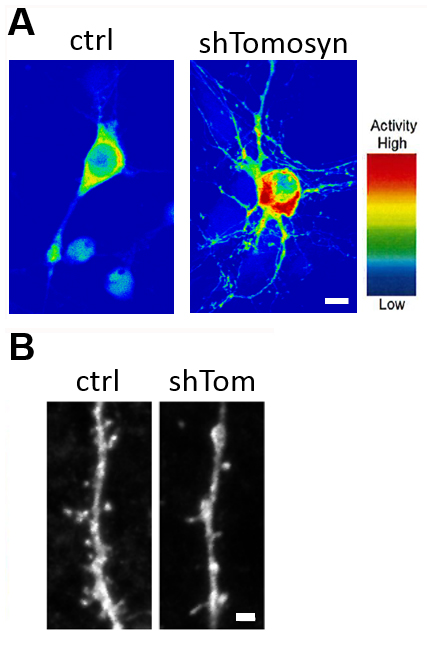
Figure 2. (A) Förster Resonance Energy Transfer (FRET) analysis determines Rho activity in neurons. Note that Rho activity is upregulated in neurons with the knockdown of an autism-risk gene (shTomosyn). This neuron also shows reductions in dendrite arborization and (B) dendritic spine density. Scale bar is 10 µm and 1 µm.
The cadherin pathway
The cadherin superfamily is one of the largest group of adhesion molecules, including classical type I and II cadherins, protocadherins, and atypical cadherins. In addition to mediating cell-cell contact, cadherins are often involved in differentiation, migration, neurite outgrowth, and synaptic functions in neurons. Several cadherins have been shown as autism-risk genes, although their cellular functions are not fully understood. Using sophisticated biochemical and imaging approaches to investigate samples from cultured cells, transgenic mice, human induced pluripotent stem cells (iPSCs), and postmortem brain tissues, we aim to elucidate the basic cellular functions of different autism-associated cadherins and identify the altered molecular mechanisms in ASC.
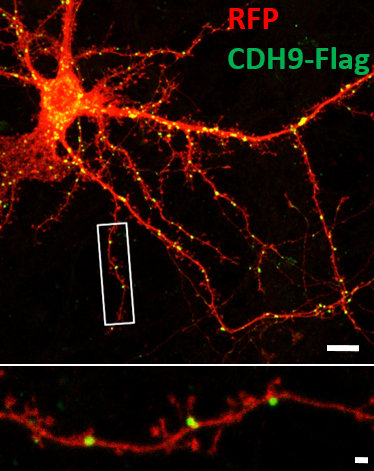
Figure 3. Cadherin-9 (green), a classical type II cadherin, exhibits punctate localization in a RFP-expressing neuron (red). A magnified image of the boxed area is shown at the bottom. Scale bar is 10 µm and 1 µm.
Lab Team
Yu-Chih Lin, Ph.D.
Investigator
Jeannine Frei, Ph.D.
Postdoctoral Research Scientist
Michaela Kilander, Ph.D.
Postdoctoral Research Scientist
Robert Niescier, Ph.D.
Postdoctoral Research Fellow
Wenjuan Shen, Ph.D.
Postdoctoral Research Fellow
Contact Information
Yu-Chih Lin, Ph.D.
Investigator
Laboratory of Neuronal Connectivity
Program in Neuroscience
Hussman Institute for Autism
801 W. Baltimore Street / Suite 301
Baltimore, MD 21201
443-860-2580 ext. 733
yclin@hussmanautism.org
